Ionization energy: is the amount of energy required to remove the most loosely bound electron
(highest energy electron) from a gaseous atom. It is an endothermic process.
First ionization energy: It is the energy needed to remove one electron from a neutral gaseous
atom to form a single positively charged gaseous ion.
Second ionization energy: It is the energy needed to remove one electron from a single positively charged gaseous ion to form a +2 charged gaseous ion.
General equation for ionization energy:
Equation for nth ionization energy:
The second ionization energy for any element is always greater than the first ionization energy.
The second ionization involves the removal of an electron from positively charged ion where the
same nuclear charge is attracting smaller number of electrons, so electrons are harder to remove and
more energy is needed to remove the electrons.
Second IE is always larger the first.
Core electrons are much harder to remove than valence electrons.
Generally, as we go down a group, the nuclear charge increases and the shielding effect
increases so the valence electrons are more shielded from the full nuclear charge and it is easier to
remove electrons. Therefore, as we go down a group ionization energy generally decreases.
Generally, as we go across a period from left to right, for the same shielding effect, the
nuclear charge increases, so the nuclear attraction to the valence electrons is higher and it is harder to
remove the electrons. Therefore, as we go across a period, ionization energy increases.
Exception 1 to trend of ionization energy: First ionization energy of group 3 elements is less than first ionization energy of group 2 elements

Exception 2 to trend of ionization energy: First ionization energy of group 6 elements is less
than first ionization energy of group 5 elements
IE1 of oxygen is less than IE1 of nitrogen. Explain
Elements with low ionization energy tend to lose electrons while elements with high ionization energy tend to share or gain electrons.
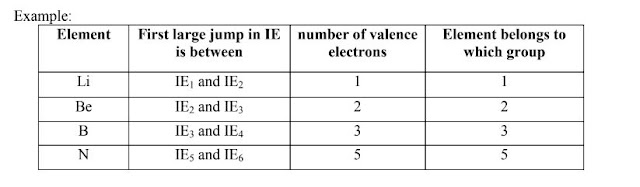
Ionization energy can be used to determine the group the element belongs to as well as the number of valence electrons.
By examining the table below, we notice that the first large jump between two successive ionization
energies can be used to determine the number of valence electrons.
(highest energy electron) from a gaseous atom. It is an endothermic process.
First ionization energy: It is the energy needed to remove one electron from a neutral gaseous
atom to form a single positively charged gaseous ion.
Second ionization energy: It is the energy needed to remove one electron from a single positively charged gaseous ion to form a +2 charged gaseous ion.
General equation for ionization energy:
Equation for nth ionization energy:
The second ionization energy for any element is always greater than the first ionization energy.
The second ionization involves the removal of an electron from positively charged ion where the
same nuclear charge is attracting smaller number of electrons, so electrons are harder to remove and
more energy is needed to remove the electrons.
Second IE is always larger the first.
Core electrons are much harder to remove than valence electrons.
Generally, as we go down a group, the nuclear charge increases and the shielding effect
increases so the valence electrons are more shielded from the full nuclear charge and it is easier to
remove electrons. Therefore, as we go down a group ionization energy generally decreases.
Generally, as we go across a period from left to right, for the same shielding effect, the
nuclear charge increases, so the nuclear attraction to the valence electrons is higher and it is harder to
remove the electrons. Therefore, as we go across a period, ionization energy increases.
Exception 1 to trend of ionization energy: First ionization energy of group 3 elements is less than first ionization energy of group 2 elements
Exception 2 to trend of ionization energy: First ionization energy of group 6 elements is less
than first ionization energy of group 5 elements
IE1 of oxygen is less than IE1 of nitrogen. Explain
Elements with low ionization energy tend to lose electrons while elements with high ionization energy tend to share or gain electrons.
Ionization energy can be used to determine the group the element belongs to as well as the number of valence electrons.
By examining the table below, we notice that the first large jump between two successive ionization
energies can be used to determine the number of valence electrons.
















No comments:
Post a Comment