Basic Questions
Sample Questions
Points Discussed
189. Condensed phases of matter are solid and liquid.
190. Gaseous elements (under room conditions) are found at the top right hand side of the Periodic Table. SQ1
SQ1 Where in which part(s) of the periodic table do you find the elements that are gaseous under room conditions?
Examples of element that are found as gases are:
Hydrogen, H2 Helium, He Radon, Rn
Nitrogen, N2 Neon, Ne
Oxygen, O2 Argon, Ar
Fluorine, F2 Krypton, Kr
Chlorine, Cl2 Xenon, Xe
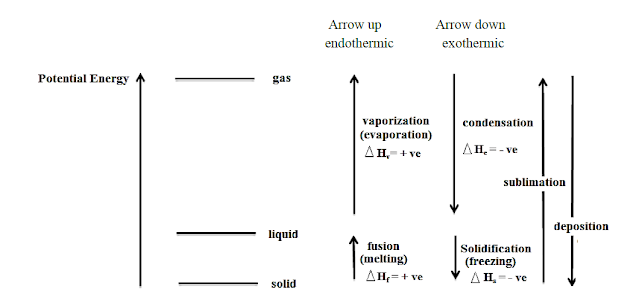
Study the following Potential Energy diagram:
The exothermic reactions are: condensation, freezing (solidification) and deposition.
The endothermic reactions are: vaporization (evaporation), melting and sublimation
Endothermic reaction: a reaction that consumes energy
Exothermic reaction: a reaction that releases energy.
191. One gram of steam, H2O (g) causes more severe burns than one gram of water, H2O(l) at 100oC. At the same temperature, both have the same average kinetic energy but steam has a higher potential energy than water.
192. A volatile liquid is a liquid that evaporates at room temperature. A liquid with a low boiling point is easy to vaporize. SQ3
SQ3 A liquid is called volatile if
a) it burns spontaneously in air.
b) it reacts explosively with oxygen.
c) it readily evaporates at room temperature.
d) it boils when heated in air.
e) it catches fire if heated sufficiently in air.
Compounds A, B and C have the following boiling points: -15℃, 60℃ and 38℃ respectively. List the above compounds from the least to the most volatile.
193. Vapor pressure of a liquid: is the pressure of the gas above the liquid with which it is at equilibrium (Both liquid and gas exist indefinitely). SQ9
SQ9 When a liquid is in contact with its vapor at equilibrium at a constant temperature, thepressure exerted by its vapor is called
a) the total pressure.
b) the partial pressure of the liquid.
c) the liquid pressure of the vapor.
d) the vapor pressure of the liquid.
e) the atmospheric pressure.
194. Vapor pressure of a liquid in a sealed container depends on temperature of the flask. As the temperature increases the vapor pressure of a liquid increases. SQ2, 4
SQ2 Some liquid is found at the bottom of a sealed flask containing air. Which is true about its vapor pressure?
a) It depends upon the amount of liquid present at the bottom.
b) It depends upon the volume of the flask.
c) It depends upon the temperature of the flask.
d) It depends upon the pressure of the gas in the flask.
e) It depends upon the pressure of average molar mass of the gas in the flask.
SQ4 How does the vapor pressure of a liquid vary with temperature?
195. At the boiling point, the temperature of a pure substance stays constant as the liquid is being heated until all the liquid changes into gas. The heat given to the liquid causes more liquid to change into gas. SQ5, BQ1a
SQ5 When a pure substance at its boiling point is heated, how does its temperature change? What happens to the heat given to the liquid?
BQ1 A liquid is heated at its boiling point. Although energy is used to heat the liquid, its temperature does not rise. Explain.
196. Molar heat of vaporization is the minimum energy required to change one mole of a substance from liquid to gas at the same temperature. SQ7
SQ7 Define the molar heat of vaporization.
197. General equation for Molar heat of vaporization: X (l) + heat ⇌ X (g)
198. General equation for Molar heat of condensation: X (g) ⇌ X (l) + heat
SQ6
1. When a liquid evaporates, it
a) gives energy to the surroundings.
b) takes energy from the surroundings.
c) neither takes nor gives energy to the surroundings.
2. Which of these equations is correct?
a) H2O(l) + 42 kJ → H2O(g)
b) H2O(g) → H2O (l) + 42 kJ
c) H2O(g) + 42 kJ → H2O (l)

199. In general, a substance that has a higher boiling point is expected to have a higher molar heat of vaporization. SQ8, BQ1b, c
SQ 8
a) In general, what can you say about the molar heats of vaporization of pure substances with higher boiling points? (Study table 6.1)
b)The boiling points of five substances, in ◦C are given in the table below

Based on this data alone, which of the above substances is expected to have the highest molar heat of vaporization?
Typical exercise: Find the heat released by a given mass from the heat of vaporization
If the molar heat of vaporization of water is 42 kJ/mole. What is the amount of heat needed to
BQ1b What maximum amount of heat can you lose as 4.5 gram of water evaporates from your skin? [Molar heat of vaporization of water = 42 kJ/mol]
BQ1c What is the minimum mass of water that should evaporate from your skin to lose 8.4 kJ?
NOTES:
1. Vapor pressure and temperature are proportional NOT directly proportional.
2. At the same temperature, the vapor pressure is the SAME.
3. For the same liquid, the only factor affecting the pressure of the liquid is the temperature.
200. Minimum conditions for liquid molecules to vaporize:
1) Molecules are supposed to be on the surface.
2) Molecules are supposed to have an average kinetic energy greater than the energy keeping the molecules in the liquid state.
Water has a vapor pressure of 17.5 mmHg at 20oC. Which of the following will increase the vapor pressure of water?
a) Transferring water to a larger container.
b) Cooling water to 10oC
c) Taking the container to the top of the mountain.
d) Heating the water to 32oC
Note: If the question asks “what changes the vapor pressure” then the correct answer is b and d
Date 25-08-20 | Level L | 5
201. Boiling point: is the temperature at which the liquid vaporizes anywhere in the solution.
202. At the boiling point:
a. Vapor pressure is equal to the surrounding pressure.
b. Bubbles of vapor can form anywhere within the liquid.
c. Molecules escape from the surface of the liquid to enter the gas phase as vapor (this also happens at room temperature).
d. With increasing altitude, atmospheric pressure decreases and so does boiling point.
SQ10 At the boiling point:
a) can molecules escape from the surface of a liquid to enter the gas phase as vapor?
b) what is the relationship between the vapor pressure and the atmospheric pressure?
c) can bubbles of vapor form anywhere within the liquid?
SQ11
1. In general, a liquid boils when
a) its vapor pressure is 1 atmosphere.
b) its vapor pressure is 760 mm Hg.
c) its temperature is 100°C.
d) its vapor pressure equals the surrounding pressure.
e) bubbles form only on the sides of its container.
2. When the surrounding pressure is 740 mmHg, water boils at 97◦C while ethanol boils ate 76◦C. Which compound has the higher vapor pressure?
SQ12 When will the boiling point of water be highest?
a) At sea level.
b) On the hottest day.
c) On the coldest day.
d) At the top of Mount Everest.
e) When it is boiled in a metallic kettle.
If you have 2 containers, one with alcohol and the other with water. Alcohol boils at 78.5oC while water boils at 100oC. If the atmospheric pressure is 750 mmHg, what is the vapor pressure of each liquid? Explain
203. Normal boiling point: is the temperature at which the vapor pressure is exactly 1 atm or 760 mmHg. SQ13
SQ13 The normal boiling point of a liquid is defined as the temperature at which
a) its vapor pressure is constant.
b) its vapor pressure is 760 mm Hg.
c) its temperature is 100°C.
d) its vapor pressure equals the surrounding pressure.
e) bubbles form on the sides of its container.
204. Molar heat of fusion: is the energy required to change one mole of a substance from solid to liquid at the same temperature and constant pressure. SQ14, 16
SQ14 When a solid melts, it
a) gives energy to the surroundings.
b) takes energy from the surroundings.
c) neither takes nor give energy to the surroundings.
SQ16 Define “molar heat of fusion”.
205. Molar heat of fusion is less than the molar heat of vaporization. Molar heat of vaporization of water is 7 times molar heat of fusion of water. SQ15
SQ15 If H2O(l) + 42 kJ → H2O(g), which of these equations is most likely to be correct?
a) H2O(s) → H2O (l) + 42 kJ
b) H2O (s) + 6.0 kJ → H2O (l)
c) H2O(s) + 42 kJ → H2O (l)
d) H2O(l) + 42 kJ → H2O (s)
206. In general, a pure substance that has a high melting point is expected to have a high molar heat of fusion. SQ17
SQ17
a) How does the molar heat of fusion of pure substances vary with melting point?
b) Based on this data alone, which of the above substances is expected to have the highest molar heat of fusion? Substance A (the higher the melting point, the higher the molar heat of fusion) The melting points of five substances, in C are given in the table below
Based on this data alone, which of the above substances is expected to have the highest molar heat of fusion?
Applications:
1. Exercise 6 page 8
How much heat would 180g of water release when they change from water at 0ºCto ice at 0°C?
2. Exercise 5 (b, c) page 8
Because of its excellent heat conductivity, liquid sodium has been proposed as a cooling liquid for use in nuclear power plants.
b) How much heat would be absorbed per kilogram of sodium to melt the solid when the cooling system is put in operation?
c) How much heat would be absorbed per kilogram of sodium if the temperature rose too high and the sodium vaporized?
Use the data in Tables 6.1 and 6.2.
207. Comparison between the energy of the molecules on the three states of matter.
Highest PE in gases and lowest PE in solids. The PE of liquid is less than in gases and more than in solids. SQ18, 19

208. Average KE is the same in all the states of matter at the same temperature. SQ18
What factor affects average KE?
SQ18 At the same temperature which molecules are on the average likely to have the highest kinetic energy?
a) Gaseous molecules.
b) Solid molecules.
c) Liquid molecules.
d) Heaviest molecules.
e) Molecules of all states will have the same average KE.
SQ19 If you define the overall energy of a molecule to be the sum of its kinetic energy and potential energy, which molecules on the average have the highest overall energy?
a) Gaseous molecules
b) Solid molecules
c) Liquid molecules
d) Heaviest molecules
e) All have the same average total energy
209. During melting, the energy absorbed is stored as PE. This stored energy is used to change state form solid to liquid and not to raise the temperature. Therefore, the temperature stays constant.
210. Heating curve:
As temperature increases: KE increases, PE stays the same
At constant temperature: KE stays the same, PE increases.
BQ2
211. The average kinetic energy is directly proportional to absolute temperature. At constant temperature, average kinetic energy remains the same.
212. If a molecule on the surface of a liquid is given a sufficient push from below, it may be able to escape and join the gaseous state.
213. The molecule that leaves the surface jumps out with a lot of kinetic energy, energy that is stored as potential energy.
214. The average kinetic energy of the remaining molecules decreases. The liquid cools down.
SQ20
SQ20 When some of the liquid in a flat dish evaporates,
a) the remaining liquid becomes hotter because evaporation needs heat.
b) the remaining liquid becomes cooler because the molecules that escape take energy with them.
c) the molecules that evaporate become colder than the remaining liquid
d) the molecules that evaporate become hotter than the remaining liquid
e) the average kinetic of the remaining molecules stays the same.
215. When a liquid evaporates its vapor pressure starts to rise. Eventually, molecules come back and join the liquid at the same rate they are escaping it. The net amount of liquid molecules
and gaseous molecules becomes constant. No more cooling takes place because the rate at which molecules leave and cool the liquid is equal to the rate at which the molecules come back and heat the liquid. Equilibrium is reached. It is this “maximum” or “equilibrium” pressure that is called the vapor pressure of the liquid at the given temperature.
216. When we sweat on very humid days our bodies do not cool down.
217. The vapor pressure increases with increasing temperature.
218. When the vapor pressure equals the atmospheric pressure, the liquid boils.
219. Sometime a liquid is heated to a temperature above its boiling point and it does not boil. The liquid is said to be superheated. This is dangerous because any small agitation will cause the liquid to evaporate explosively spraying all with very hot liquid.
220. To prevent superheating in the laboratory, we add a few boiling chips to a liquid before boiling it.
Part 3
221. Solution: is a homogeneous mixture SQ21
SQ21 Define solution.
222. Types of solution: SQ22
SQ22 Which of the following is/ are a solution? Sea water, iron, Jewelry (Gold), Cola drink (freshly open), Milk, Brass, Steel knife, Mud, Air, Liquid mercury
223. Differences between solution and a pure substance with respect to phase change:

224. If we collect the vapor from a boiling aqueous solution, condense it and boil it again it will boil at 100⁰C.
225. If we collect the crystals from a freezing aqueous solution, melt it and freeze it again it will freeze at 0⁰C. SQ 23, 24, BQ3, 4
SQ23
1. A change of phase of a solution results in components
a) that are the same as the starting solution.
b) that are different from the starting solution.
c) that are pure elements.
2. A solution is _______________ with respect to appearance and _______________ with respect to phase
SQ24 At the same pressure, the boiling and melting points of a solution
a) are always constant.
b) are different for the same composition.
c) are different for different compositions, where the higher the concentration of the solution, the higher the boiling point and the lower the freezing point.
BQ3
a) How can you obtain pure water from sea water by freezing?
b) How can you verify that a given liquid is pure water?
c) List the properties of a solution you would expect to vary as the concentration of the solute varies.
BQ4 Which of the following statements about seawater is false?
a) Seawater boils at a higher temperature than pure water.
b) Seawater freezes at a lower temperature than pure water.
c) The boiling point rises as the liquid boils away.
d) The melting point falls as the liquid freezes.
e) The density is the same as that of pure water.
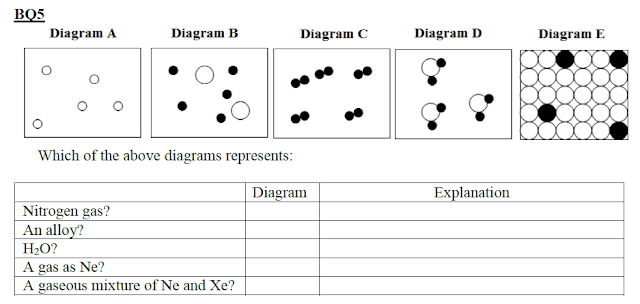
226. Diagrammatic representations of elements compounds in the 3 states of matter. BQ5
227. Demonstration: filtration SQ25
SQ25 How would you obtain dry sand and dry salt from a mixture of sand and salt? Describe clearly all the steps you follow.
228. Selective solubility SQ26
SQ26
1. AgCl does not dissolve in water, but Na2CO3 does. How would you separate a mixture of the two?
2. Sugar dissolves in water and alcohol, and salt dissolves in water but not in alcohol. How do you separate a mixture of salt and sugar?
229. Alcohol is flammable, therefore it cannot be heated directly. To heat alcohol, we should use a steam bath or an electric heater.
230. If you need to collect sugar from sugar alcohol solution heat the solution using an electric heater to crystallization point. Leave the solution to cool and crystals to form. Filter off the crystals. SQ27
SQ27 It is required to heat a beaker containing some alcohol. How should this be done SAFELY?
231. Demonstration: Sublimation: Examples of solids that can sublime at room temperature:
1) Solid iodine, I2 (s)
2) Dry ice or solid carbon dioxide CO2 (s)
3) Any ammonium compound as ammonium chloride, NH4Cl and ammonium bromide, NH4Br SQ28, 29
SQ28 What does it mean to say that a substance sublimes?
SQ29 Which mixture is easy to separate by sublimation?
a) Salt + sand
b) Salt + sugar
c) Salt plus ammonium chloride
d) Nitrogen and oxygen liquids
e) Ammonium chloride dissolved in water.
f) Sodium chloride (table salt) and iodine
g) Dry ice and table salt
232. Demonstration: Simple Distillation SQ30, 31
SQ30 Which mixture is easy to separate by distillation?
a) Salt + sand
b) Salt + sugar
c) Salt plus ammonium chloride
d) Nitrogen and oxygen gases
e) Calcium chloride dissolved in water.
SQ31
a) Name all the apparatus needed to perform a distillation
b) Name the liquid collected at the end of distillation?
233. Demonstration: Fractional distillation. Discuss briefly: fractional distillation of liquefied air and fractional distillation of crude oil. SQ32, 33
SQ32 Which mixture is easy to separate by fractional distillation?
a) Salt + sand
b) Salt + sugar
c) Salt plus ammonium chloride
d) Nitrogen and oxygen liquids
e) Calcium chloride dissolved in water.
f) Alcohol + water (where alcohol is obtained as a distillate from the mixture)
SQ33 When do we use a fractionating column?
234. Demonstration: Separating funnel SQ34
SQ34 Which mixture is easy to separate by using a separating funnel?
a) Salt + sand
b) Water and alcohol
c) Salt plus ammonium chloride
d) Nitrogen and oxygen liquids
e) Water and oil
235. Adsorption: means sticking to the surface. SQ35, 36
SQ35 What is adsorption?
SQ36 Which mixture is easiest to separate into components by adsorption?
a) Brewed tea
b) Sea water
c) Sand and salt
d) Blue copper sulfate solution
e) Yellow potassium chromate solution.
f) a solution of brown sugar
236. Adsorption: sticking of the particles of one material on the surface of another. Examples of adsorbing substances:
Silica gel: adsorbs water vapor
Charcoal: adsorbs gases with strong odor and removes colored impurities from a solution
SQ37
SQ37 Which of the following is a good adsorbing agent?
a) Sponge
b) Charcoal
c) Sand
d) Silica
e) Filter paper.
237. Demonstration: Chromatography SQ38
It is the technique used to separate different compounds, especially those that can be easily destroyed by heat or chemicals.
It can be used to separate colored components as:
1) Green liquid obtained by squashing green leaves.
2) Black ink
The property that carries the liquid up the paper is capillary action.
Study of a chromatography paper after it dries:
Chromatography could be used to check the purity of a dye as well as a separation technique.
Dyes 1 and 2 are soluble pure substances . The spot moved up the paper with the solvent and did not split.
Dyes 3-6 are mixtures of soluble components. The spot moved up with the solvent and split into more than one spot. The greater the solubility of a component the greater is its Rf value the larger is the separation from the base line.
Dyes 2 and 5, 3 and 5, 3 and 6 have a common component same Rf value. Rf value is a physical constant.
If a dye sticks to the x on the base line this dye is considered to be insoluble in the solvent.
SQ38
SQ38 Which mixture is easiest to separate into components by chromatography?
a) Water and alcohol
b) Sea water
c) Green liquid obtained by squashing green leaves
d) Blue copper sulfate solution
e) Yellow potassium chromate solution.
f) Different dyes present in red ink
238. Demonstration: Crystallization SQ39
SQ39 Crystallization is suitable to obtain certain solids from their solution. Give an example.
Note: Students should study sec 3 from text book
Applications on sec 6.3
1. Chapter review Q 17 page 54
Complete the following statements:
a) A __________ is the solid left on the filter paper.
b) The __________ is the liquid that passes through the filter paper.
c) The process of __________ a liquid and then __________ the vapor is known as __________
d) __________ is the process used to separate dry ice from table salt.
e) __________ is the process used to separate sugar from salt.
2. Chapter test Q 8 page 59
a) From the list below choose the process shown in each diagram.
Chromatography distillation crystallization filtration
b) Choose which process A, B or C, you could use to separate pure water from sea water.
c) Choose which process A, B or C, you could use in food analysis to show that a bottle of fruit squash may contain several water-soluble dyes.
3. Chapter test Q 10 page 60
Rock salt contains insoluble solids, and the soluble salt, sodium chloride. The following processes are needed to separate sodium chloride from rock salt.
Evaporation Crystallization Addition of water Filtration Stirring
Put each process in the correct order for separating sodium chloride from rock salt.
4. Chapter test Q 11 page 60
You are given some mixtures below. Name the method you would think the most appropriate to obtain water from each mixture.
a) Salt + Water
b) Alcohol + Water
c) Sand + Water
d) Water vapor + Air
239. An aqueous solution is one in which the solvent is water.
240. Salt and water is an example of aqueous solutions where the solute is a solid.
241. Alcohol and water is an example of aqueous solutions where the solute is a liquid.
242. Ammonia and water is an example of aqueous solutions where the solute is a gas SQ40
SQ40 a) What are aqueous solutions?
b) Can you have aqueous solutions where the solute is a solid, a liquid or a gas?
c) Give an example of each case.
Part 4
243. Concentration: relative amounts of solute and solvent.
244. Molar concentration (Molarity): is the number of moles of solute per liter (dm3) of solution. (the relative amounts of solute and solution) SQ 41
0.5 M of solution means 0.5 moles of solute in 1 dm3 solution
1.0 M of solution means 1.0 moles of solute in 1 dm3 solution
5.0 M of solution means 5.0 moles of solute in 1 dm3 solution
SQ 41
a) What does the term 'the molar concentration of a solution' refers to?
b) Is this the same thing as molarity?
c) What does it mean to say that the molar concentration of a sugar solution is 0.25 M?
245. Concentration of a given solution does not change if solution is split into fractions. SQ 42, Ex21 p.25
A 2 L bottle of 0.35 M solution is split into ten containers of 100ml capacity. What is the concentration of the solution in each of the new containers?
a) 0.75 M
b) 0.0035 M
c) 2.0 M
d) 0.35 M
e) 100 M
SQ42 Two liters of 1.0 M NaCl solution is prepared in a flask. 500 ml of solution is poured out of the flask into a beaker. What is the concentration of the salt solution in the beaker? 1.0M
Ex 21 page 25
What do we mean by a 1.5 M solution?
Which has a higher concentration, 20 ml of 1.5 M solution or 200 ml of the same solution?
246. Relationships between n, V, C and m, M, V, C: SQ 44, BQ 6, 7
SQ44 0.15 dm3 of 2.0 M NaOH solution is to be prepared in a flask.How many moles of NaOH are required?
BQ6
a) If 1.0 mole of a solute is dissolved in enough water to make 2 dm3 of solution, what is C, the molar concentration of this solution?
b) Pour the 100 cm3 of 4.0 M salt solution into a clean volumetric 500 cm3 flask. Add enough water to fill the flask up to the etched mark. What is the concentration of the salt solution in the new flask?
c) Given 2.0 dm3 of 1.5 M solution. How many moles of solute are in the solution?
d) How many moles of sodium chloride, NaCl, are dissolvedin 50 cm3 of 4.0 M solution?
BQ 7
a) What mass of ammonium chloride, NH4Cl, are present in 0.20 dm3 of a 0.50 M NH4Cl solution?
b) What volume of a 0.250 M K2CrO4, solution contains 38.8 grams of K2CrO4?
c) 2.00 dm3 of a 1.00 M solution contain 73 g of an acid X. What is the molecular mass of X?
247. Preparing solutions with given concentrations: SQ 43, CRQ 22 (a), page 54
248. Dilution of solution: If a solution is diluted by adding water
Number of moles in concentrated solution = number of moles of dilute solution. CV = C’V’
Upon dilution, volume of water needed for dilution = V dilute solution – V concentrated solution
1) Pour the 100 cm3 of 4.0 M salt solution into a clean volumetric 500 cm3 flask. Add enough water to fill the flask up to the etched mark. What is the concentration of the salt solution in the new flask?
2) 200 cm3of 0.40 M NaCl solution was poured into a 500 cm3 beaker. Water was added till the etched mark.
a) What is the new concentration?
b) What is the volume of water needed for dilution?
3) You are given 100 cm3 of 0.50 M HCl, how much water must you add to reduce the concentration to 0.10M.
4) To what volume must 50.0 cm3 of 3.50 M H2SO4 be diluted in order to make a 2.00 M H2SO4?
249. To find [solutes] after mixing two or more solutions:
Step 1: Calculate the number of moles of each solution
Step 2: Calculate the total volume
Step 3: Find new concentration.
What is the concentration of NaCl in a solution prepared by mixing 20 cm3 of 0.40 M NaCl and 30 cm3 of 0.10 M NaCl solutions?
250. Mixing equal volumes of different solutions halves the concentration of all species in the solution.
What is the concentration of NaCl and KF in a solution prepared by mixing 20 cm3 of 0.40 M NaCl and 20 cm3 of 0.10 M KF solutions?
251. Saturated solution: is a solution that has dissolved in it the maximum amount of solute it can hold at a given temperature and is in contact with the undissolved solute. In simple words, a saturated solution is a solution in which no more solute can dissolve. SQ 45
SQ 45 What is meant by a ‘saturated solution’?
252. Solubility: it is the concentration of a saturated solution at a certain temperature. SQ 46
Soluble solubility > 0.1 M
Slightly soluble solubility < 0.1 M
Very slightly soluble solubility < 10-3 M
Insoluble or negligible solubility solubility is low
SQ 46 What is meant by the term ‘solubility’?
Part 5
253. SELF STUDY: the electric model of atoms, direction of electric current, meaning of fundamental property, effect of distance on electric force. SQ 47 – 50
SQ47 According to our model of electricity, which of the following is wrong?
a) Each electron carries a charge of –1.
b) Each nucleus carries a charge of +1.
c) If a neutral atom has 5 electrons going around it, then the charge on its nucleus is +5 units.
d) If a neutral atom loses 2 electrons the total charge on it becomes +2 units.
e) If a neutral atom gains 2 electrons the total charge on it becomes +2 units.
SQ48 The direction of the current in a wire connected to a battery is always:
a) away from the positive terminal and towards the negative.
b) away from the negative terminal and towards the positive.
c) the same as the direction of flow of electrons.
d) the same as the direction of flow of protons in the wire.
e) depending on which end of the wire the battery is connected to.
SQ49 When scientists call a property "fundamental", what do they mean?
SQ50 Experiments support the generalization that the magnitude of the electric force between two charged small spheres is dependent on the distance between their centers. What is this dependence?
254. Conductor: a material that completes an electric circuit
255. Electric current: is the movement of electric charge. SQ 52
SQ52 What is an electric current?
256. Electrolyte: a substance that dissolves in water producing electrically conducting solutions, example: aqueous solution of sodium chloride.
257. DEMONSTRATION for conductors and non-conductors. SQ51
SQ51 Which of the following liquids completes an electric circuit?
a) Distilled water
b) An aqueous solution of sodium chloride
c) An aqueous solution of sugar
258. Non-electrolyte: a substance that dissolves in water producing electrically non-conducting solutions, example aqueous solution of sugar and distilled water. Water itself is a bad conductor of electricity. When small quantities of a certain substance are dissolved in water, water becomes a good conductor of electricity.
259. Examples of electrolytes:
All acids are electrolytes e.g acetic acid CH3COOH, sulphuric acid H2SO4...
All ionic compounds are electrolytes e.g NaCl, CaCl2, MgBr2....
260. Brief introduction to section 6.6.3
261. Ions: are charged particles( charged atoms or groups of atoms) SQ54
SQ54 Define “ion”.
262. Cations: positively charged ions (usually metal ions eg Na+, Ca2+..) SQ65
SQ65 Define cation.
263. Anions: negatively charged ions ( non-metal ions, eg Cl-, CO32-) SQ64
SQ64 Define anion.
264. Ionic compounds: are compounds in which oppositely charged ions are held together strongly by an electrostatic force of attraction. They are formed from the reaction between a metal and a non-metal. Examples of ioninc compounds are sodium chloride (table salt), NaCl, calcium chloride, CaCl2, and silver nitrate, AgNO3.
265. Sodium chloride NaCl is made from the combination of sodium ions Na+ and chloride ions Cl- in a ratio of 1:1. When one mole of sodium chloride dissolves in water it provides the solution with two moles of ions( 1mole of sodium ions and 1 mole of chloride ions) as per the equation: NaCl(s) →Na+(aq)+Cl-(aq) SQ53
SQ 53 When NaCl dissolves in water, we have (identify the correct choices)
a) sodium ions, represented by Na+(aq)
b) chloride ions, represented by Cl–(aq)
c) solid sodium chloride forming ions as follows: NaCl(s) →Na+(aq)+Cl-(aq)
266. Calcium chloride is made from the combination of calcium ions Ca2+ and chloride ions Cl- in a ratio of 1:2. When one mole of calcium chloride dissolves in water it provides the solution with three moles of ions( 1mole of calcium ions and 2 moles of chloride ions) as per the equation: CaCl2(s) → Ca2+(aq) + 2Cl–(aq). SQ56
SQ 56 Why does aqueous CaCl2 solution conduct electricity but sugar in water does not?
Which of the following is a part of the explanation?
a) Sugar solution in water forms only one type of ion: Sugar(aq).
b) Calcium chloride forms two types of ions: CaCl2(s) → Ca2+(aq) + 2Cl–(aq).
c) Calcium chloride provides Ca+(aq) and Cl–(aq).
267. Silver nitrate AgNO3 is made from the combination of silver ions Ag+ and nitrate ions NO3- in a ratio of 1:1. When one mole of silver nitrate dissolves in water it provides the solution with two moles of ions( 1mole of silver ions and 1 mole of nitrate ions) as per the equation: AgNO3 (s) → Ag+(aq) + NO3-(aq) SQ57
SQ 57 One mole of silver nitrate, AgNO3, in water provides how many moles of ions?
a) One mole of Ag+(aq) and one mole of N–.
b) One mole of Ag+(aq) and three moles of NO3− (aq).
c) One mole of Ag+(aq) and four moles of negative ions (aq).
d) One mole of Ag+(aq) and one mole of negative nitrate ions, NO3− (aq).
e) Five moles of ions.
268. Sodium chloride and all other ionic compounds are electrolytes because they provide the solution with freely moving ions which can carry electric charge.
269. An electric current flows when we have a complete circuit. Current flows outside the battery from the positive terminal to the negative terminal. If an ionic solution is connected to a battery, all positive ions move in the direction of the current, away from the positive electrode and towards the negative electrode. All negative ions move in a direction opposite to the current, away from the negative electrode and towards the positive electrode. SQ55
SQ 55 Which of the following points is necessary for an electric current to flow through anaqueous solution?
a) An electric current flows when we have a complete circuit.
b) Current flows outside the battery from the positive terminal to the negative terminal.
c) Inside the battery, the current moves from the positive side to the negative side.
d) In an ionic solution connected to a battery, all positive ions move in the direction of the current, away from the positive electrode and towards the negative electrode. All negative ions move in a direction opposite to the current, away from the negative electrode and towards the positive electrode.
e) All the above are correct
270. Not all ionic compounds are soluble in water and ionize completely in it. Examples include: calcium carbonate, silver chloride, silver bromide, silver iodide and barium sulphate. SQ58
SQ 58 Which solid is not soluble in water?
a) Silver chloride, AgCl.
b) Silver nitrate, AgNO3.
c) Sodium chloride, NaCl.
d) Calcium chloride, CaCl2.
e) Ammonium chloride, NH4Cl.
271. Memorize name and formulae of cations and anions listed p. 47 part2 textbook. SQ66
SQ66 Which of the following is FALSE?
a) Cations that have a charge of 2+ are magnesium, calcium, barium and lead.
b) Anions that have a charge of 2– are sulfate, carbonate, and chromate.
c) The cation that is made of several atoms is ammonium.
d) SO23− is the sulfate ion.
e) The dichromate ion is Cr2O72−.
272. Naming ionic compounds: cation is always named first followed by the anion.
BQ9, SQ67
BQ9 Name the following:
SQ67 Which salt is named or written incorrectly?
a) K2Cr2O7 is potassium dichromate.
b) NH4Cl is chloride ammonium.
c) Lead sulfate is PbSO4.
d) K2CO3 is potassium carbonate.
e) Barium hydroxide is Ba(OH)2.
273. Writing the formulae of ionic compounds BQ10
BQ10 Write the formulae of the following:

274. Properties of an ionic solid: ionic solids have high mpt and bpt, do not conduct electricity when solid, conduct electricity when molten or aqueous. Ionic solids form clear crystals. SQ63
SQ 63
1. What can you say about the following properties of ionic solids, as NaCl?
a)
melting an boiling points
b)
Electrical and thermal conductivity
c)
Formation of crystals
2. Which is FALSE about NaCl?
a) At room temperature, it is made of NaCl(s) molecules.
b) At room temperature, it conducts electricity
c) In the crystalline state, every sodium ion is surrounded by six chloride ions.
d) Above 808°C, it is a liquid that conducts an electric current.
e) NaCl is its empirical formula, not molecular formula.
275. Write dissociation reactions of ionic compounds in water. The equations must be balanced for atoms and for charge SQ61
276. Precipitate: is solid formed when mixing two aqueous solutions.
277. Precipitation reaction: is a reaction where a precipitate (solid) is formed. SQ59
SQ59 Precipitation in chemistry means:
a) Rain falling on a plain.
b) The reaction where a gas is formed.
c) The condensation that results when the vapors of a liquid are cooled.
d) The formation of steam.
e) The formation of solid from a solution.
278. Demonstration: Different precipitation reactions:
e.g i) silver nitrate and sodium chloride
ii) silver nitrate and sodium bromide
iii) silver nitrate and sodium iodide
iv) lead (II) nitrate and sodium sulphate
v) calcium chloride and sodium carbonate
279. Write a complete formula equation, a complete ionic equation, a net-ionic equation to represent a precipitation reaction. Use BQ8 for examples ii) iii) and iv) for applications
BQ 8
a) When solutions of sodium carbonate, Na2CO3, and calcium chloride, CaCl2, are mixed, a white precipitate of calcium carbonate, CaCO3(s) is obtained. Write balanced equations for this reaction in three different ways.
b) When solutions of silver nitrate, AgNO3, and potassium chloride, KCl, are mixed; a white precipitate of silver chloride, AgCl(s), is obtained. Write balanced equations for this reaction in three different ways.
280. Predominant ions: is that take part in the reaction
281. Spectator ions: ions that do not take part in a reaction. SQ60
SQ60 In the reaction:
The predominant reacting species is/are:
282. Molecular solids are made up of molecules. Examples of molecular solids are wax, sugar, hydrogen chloride, ammonia ...
283. Discuss properties of molecular solids: SQ62
low mpt and bpt
In general they remain molecular when dissolved in water and are non-electrolyte
In general they are non-electrolytes: sugar (s)→ sugar (aq)
Some molecular compounds like acids e.g hydrogen chloride are electrolytes:
HCl(g)→H+(aq) + Cl- (aq)
SQ62 When cold enough molecular compounds are solid. The following are molecular compound sat room temperature: CO2(g); wax(s); sugar(s); HCl(g).
Which of these statements is false?
a) Only one of the above does not dissolve in water.
b) HCl(g) dissolves in water to give an ionic solution.
c) Sugar dissolves to give a non-ionic solution.
d) All four substances have low melting points.
e) Since none of them are ionic solids, none of them form aqueous solution that conducts an electric current.
284. Other types of solids: metallic solids and network solids
285. Metallic solids: e.g Cu, Ag, Na
286. Network solids / giant molecular/ giant covalent solids are atomic solids made up of atoms joined together by strong covalent bonds. Network solids are characterized by their high melting point and boiling point. e.g Sand (SiO2) , graphite (C) and diamond(C) SQ68
SQ68 Examples of metallic and network solids respectively are
a) copper and wax.
b) salt and sugar.
c) gold and charcoal.
d) sodium and diamond.
e) mercury and string.
f) gold and sand
287. Determine [ions] present given number of moles of salt and volume of solution.
BQ11, 12, CR25
BQ11 Magnesium chloride, MgCl2, dissolves in water to form a conducting solution containing Mg2+, and that of chloride ions, Cl-.
a) Write the equation for this reaction.
b) If 0.15 mole of MgCl2 is dissolved in water and diluted to 1.5 dm3, what is the concentration of magnesium ion and that of chloride ion?
37.0 g of calcium hydroxide Ca(OH)2 are dissolved in water to make a 200 cm3 solution. what is the concentration of each ion in this solution?
A solution is prepared by diluting a 25 ml of 0.300 M solution of NiCl2 to a final volume of 500 ml. What is the concentration of: a) NiCl2 b) Ni2+ ions c) Cl- ions
288. Determine the concentration of each ion present in a solution prepared by mixing two other solutions without precipitate formation BQ12, CT 23
BQ 12 0.40 dm3 of solution which contains 0.100 mole of Na2SO4(aq), was mixed with 1.00 dm3 of solution which contains 0.100 mole of calcium chloride, ZnCl2. Calculate the concentrations of all ions in the resulting solution. Assume that volumes of these solutions are additive.
Determine the concentration of each ion in a solution prepared by mixing 20 cm3 0.10 M NaCl solution with 30cm3 0.30 M CaCl2 solution.
Calculate the concentration of all ions after mixing 0.100 moles FeCl3 and 0.100 moles of NH4Cl and dissolving them into a 1.00 dm3 solution.
289. Determine the concentration of each ion present in a solution prepared by mixing two other solutions resulting in the formation of a precipitate. Calculate the mass of precipitate formed. BQ13
BQ 13 When solutions of calcium chloride, CaCl2, and potassium carbonate, K2CO3, are mixed, the following reaction occurs:
2K+(aq) + CO32-(aq) + Ca2+(aq) + 2Cl-(aq) →CaCO3(s) + 2K+(aq) + 2Cl- (aq)
a) Rewrite the equation showing predominant reacting species only.
b) 0.500 liter of 0.400 M CaCl2 is mixed with 1.00 liter of 0.200 M K2CO3. CaCO3 has negligible solubility. Calculate the concentrations of all ions present and the mass of CaCO3 formed when precipitation stops.
c) 1.00 litre of 0.400 M CaCl2 is mixed with 1.00 litre of 0.200 M K2CO3. CaCO3 has a negligible solubility. Calculate the concentrations of all the ions present after precipitation stops.
Extra questions:
1-Determine the concentration of each ion and mass of AgCl produced in a solution prepared by mixing 20 cm3 0.10 M AgNO3 solution with 30cm3 0.20 M NaCl solution. Silver chloride is sparingly soluble.
2- Determine the concentration of each ion and mass of AgCl produced in a solution prepared by mixing 20 cm3 0.10 M AgNO3 solution with 30cm3 0.20 M CaCl2 solution. Silver chloride is sparingly soluble.
3- Determine the concentration of each ion and mass of AgCl produced in a solution prepared by mixing 20 cm30.10 M Pb(NO3)2 solution with 30cm30.10 M NaCl solution. Lead (II) chloride is sparingly soluble.






























No comments:
Post a Comment